Generating electricity from CO₂
In the lab, a marriage of unlikely opposites
Before I get to the science experiment, I should probably let readers know where I’m coming from.
I hold an apparently minority view that nature controls the climate.
I’m not particularly concerned about the level of CO₂ in the atmosphere.
Neither do I worry much about the modest increase in temperature over the past few centuries.
As a historian, I suppose I’m predisposed to take the long view of things.
A few years back, I was involved in a time-series analysis of the — admittedly imperfect — data we have for temperature going back 1,000 years.
That analysis showed that, temperature-wise, we are living on the modest upward rise of a long cycle, which is presumably solar in origin.
The upturn dates from the end of the Little Ice Age, around the year 1670.
That long cycle is further modulated by shorter cycles. I recall 247 and 66 years working well in the regression analysis.
The second number was happily close to the period of the Atlantic Multidecadal Oscillation (AMO). Schlesinger and Ramankutty’s 1994 paper in Nature described it: “An oscillation in the global climate system of period 65-70 years”.
The point of the exercise was to find any residual, a trend that remained after natural variation was subtracted.
It was hard to find: the amount of additional warming that could be attributed to CO₂ was quite small.
Indeed, when we looked more closely at CO₂, its increased concentration in the atmosphere followed the increase in temperature, not the other way round.
The Modern Warming, whatever its cause, is the best explanation of CO₂’s seemingly relentless rise. Most human-emitted CO₂ has long since entered into the vastly large natural carbon cycle of Earth.
So my weather forecast for the world of 2050 or 2100 is: cleaner, greener, and a bit warmer.
Humanity should enjoy it while it lasts. With astronomical certainty, the ice will be back.
Of course, predictions are hard to make, especially when they are about the future.
In guessing what the world will look like in 2050 or 2100, a very large wildcard is human innovation.
If we could predict innovation, we'd have to call it something else. The root of the word is novus: new.
If we look back 50 years, two climate-relevant technologies we now take for granted were effectively lab curiosities: lithium-ion batteries and solar cells.
Stanley Whittingham, a professor of chemistry at Stanford, was hired by Exxon in 1972 when the oil giant — to its credit, rarely acknowledged — decided to invest in a portfolio of non-petroleum basic energy research.
After Whittingham came up with a rechargeable lithium battery that worked at room temperature, he travelled to Exxon’s headquarters in New York.
Whittingham sold Exxon management on the battery in a meeting that lasted all of fifteen minutes. Exxon resolved to develop a commercially viable one.
The rest, as the say, is history, if a long and technically complicated one.
Fast forward to 2019 and Whittingham and two other scientists, John B. Goodenough and Akira Yoshino, were awarded the Nobel Prize for Chemistry for their work on the lithium-ion battery.
Edmond Becquerel first observed the photoelectric effect in 1839.
Patents for solar cells were filed in the 1890s.
But the cells languished as lab curiosities during the first half of the 20th century, since they worked at 1% efficiency.
In 1954, Bell Labs announced a breakthrough — which we would now consider quaint — 6% efficiency.
In 1974, solar panels were moonshot expensive, and could be found only on satellites.
The Teal Photon solar-powered calculator was introduced in 1978:
It took decades for things to change after that.
“Any sufficiently advanced technology is indistinguishable from magic,"Arthur C. Clarke wrote in 1962.
One preoccupation of medieval alchemists was the coincidentia oppositorum, the coincidence of opposites. This obsession was fueled by the supposed astrological significance of a conjunction, the coniunctio, of two different planets.
An experiment reported in Nature Communications in March 2024 tickled my fancy as a sort of coincidentia oppositorum for our times.1
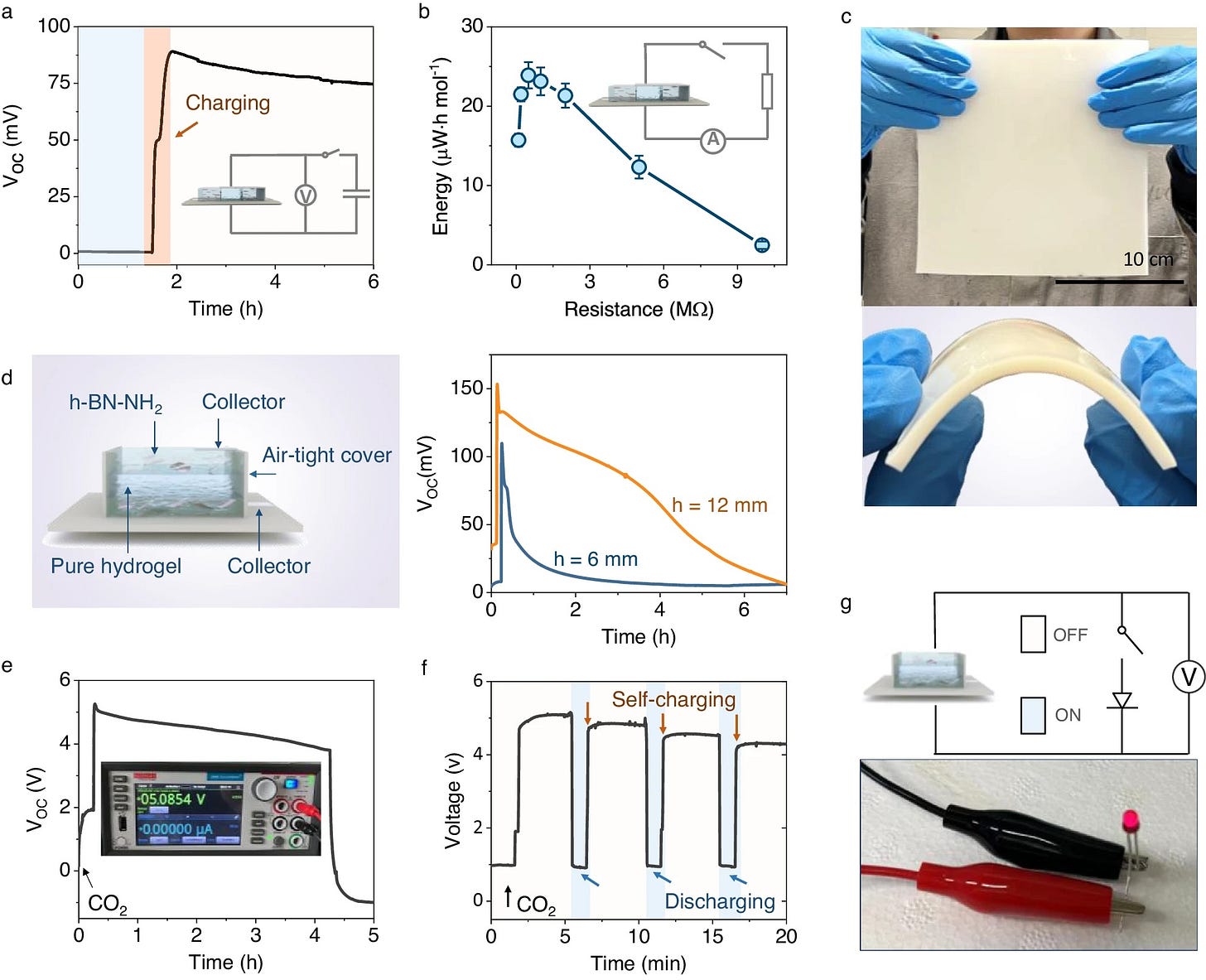
Dr. Zhuyuan Wang and his colleagues at the University of Queensland in Brisbane, Australia constructed a nanogenerator that consumes carbon dioxide and produces electricity.
This, of course, was a lab experiment, a proof of concept. Any commercial use has a long and difficult road ahead.
It does, however, do something for CO₂’s image.
“CO₂” one of Wang’s collaborators said, “can be a resource for the future.”2
Nanogenerators are an interesting class of devices made possible by advances in materials technology.
Things work differently at ‘nano’ scale.
We’re accustomed to thinking about fluids flowing through pipes and electrons running down wires.
But when the pipes are constricted enough, just a few atoms wide, there is an opportunity to control the flow.
In particular, it’s possible to differentially sort ions — charged particles — so they move in positive and negative directions.
‘Ion transportation’ may sounds arcane, but it’s routinely used in living cells, those of the human body being no exception.
And in some respects ion transportation is a more efficient process than pushing electrons down a wire.
In Wang’s experiment, ‘skeletons’ of boron nitrate only a few atoms thick were deposited on a polyamine gel, all of which was then exposed to concentrated CO₂.
Amines are a large class of chemical compounds, some of which react well with CO₂. They ‘capture’ it in a process known as adsorption. For example, monoethanolamine, MEA, is used to ‘scrub’ CO₂ from the air on submarines.
In adsorption, as opposed to absorption, molecules of a gas are captured on a surface in a thin film, a limitation being surface area.
Amines have been used to capture CO₂ chemically since the 1930s, but newly engineered materials, such polyamine nanogel particles, which can be spray-painted, hold promise to improve the process in a variety of ways.
The conventional CO₂ adsorption process produces a confusion of positive and negative ions, HCO₃⁻ and R-NH₃⁺.
Wang’s idea was to try to sort them out using the tiny channels in his boron nanosheets. It worked. The much smaller HCO₃⁻ ions roamed free, while the larger (100–300nm) NH₃⁺ molecules were trapped.
That set up what is called a diffusion current, familiar in semiconductor technology.
A diffusion current can be amplified into electricity. To prove the concept, Wang connected his cell to light up a little 1.6-volt LED (last panel):
A stack of some 50 cells could be used to charge a small battery, although as in all chemical CO₂ capture, the chemicals need to be periodically renewed. Wang was happy to get 5 adsorption-discharge cycles before his generator needed to be immersed in a strongly alkaline (pH=10) buffer solution.
There are a lot of ‘light bulb moments’ in the history of technology.
And, in fact, a lot of light bulbs, starting with Warren de la Rue’s expensive coiled platinum filament bulb in 1840. Edison’s cheaper filament, originally carbonized bamboo, came along in 1880.
Another light bulb moment I like came when the EBR-I nuclear reactor in Idaho lit up four incandescents on a wire in 1949:
Only time will tell if Wang’s LED joins that list. Electricity from carbon dioxide is, after all, just a lab novelty.
But in innovation as in climate, we need to take a long view.